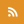Kim Kamer produced a "remarkable" thesis, the culmination of years of quiet, significant innovation
By Caitlin McDermott-Murphy
Even if you’ve heard the term “Manganism” before, it’s unlikely to provoke the same fear as cancer, for example. And it shouldn’t. Most likely, you will never develop Manganism, the Parkinson Disease-like neurological syndrome associated with manganese toxicity. Most sufferers work for years in industrial settings—think steel mills—but a handful may share a rare mitochondrial malfunction.
|
Kim will graduate in Spring 2018 |
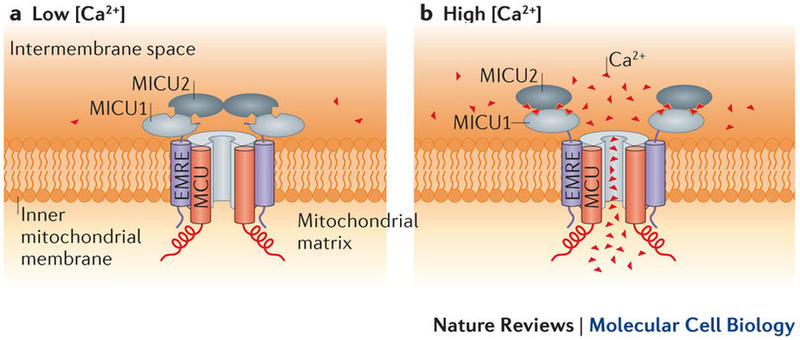
Kimberli Kamer, a graduate student in the Department of Chemistry and Chemical Biology and the Mootha Lab, dedicated her studies to investigate some of the minuscule machines that help our mitochondria function. Mitochondria, like small engines in each cell, produce energy for our bodies. And, as with all biological systems, if one of its building blocks malfunctions, our bodies can too. During her graduate studies, Kim focused her attention on a system called the mitochondrial calcium uniporter (MCU) complex. Scientists discovered the uniporter, a channel that moves calcium from the cell interior to the mitochondrion’s, decades ago. But the molecular components of this channel remained a mystery until the Mootha Lab discovered the first protein component. And, exactly how the system works—how it opens to allow calcium flow and closes to prevent overload—is still being figured out. Kim and her colleagues in the Mootha Lab are the first to explain all the molecular components under the uniporter’s umbrella. In particular, after the lab’s initial discovery of the protein MICU1, Kim helped discover MICU2, an analogous protein (pronounced “mick-you-one” and “mick-you-two”). Alone, this achievement is significant. |
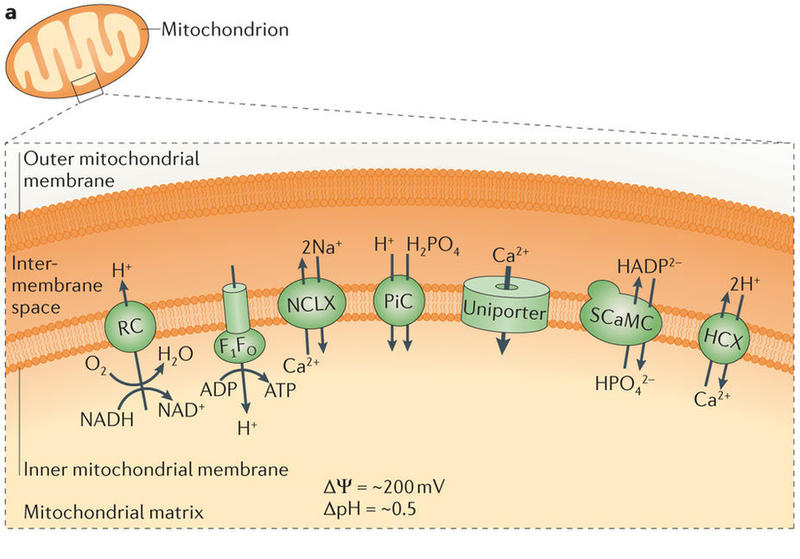
But what do these proteins do, exactly? Kim and colleagues determined that MICU1 and MICU2 work together, like gatekeepers, to control calcium flow through the channel. In their default mode, the proteins block calcium intake. Then, once calcium levels rise to a certain amount, the channel opens.
“There was a lot of confusing literature out there as to whether these proteins are inhibiting or activating the channel,” Kim explained. “[Our latest study] clarified what these proteins are doing: that they’re actually inhibiting the channel when they’re not bound to calcium and then, when calcium binds, they relieve this inhibition.”
But Kim’s thesis, “Regulation of the mitochondrial calcium uniporter by the MICU1/MICU2 complex: from biochemistry to human disease,” not only breaks down how the uniporter functions. It starts to uncover some of the rare and not so rare conditions that can result when the system fails.
|
The calcium uniporter: context and components___"The molecular era of the mitochondrial calcium uniporter"Nature Reviews Molecular Cell Biology, 2015 |
For example, in collaboration with the Chinnery Lab, the lab recently reported a neurological disorder linked to a mutation in one of the proteins, MICU1. Patients have a wide range of symptoms and, according to Kim, report “both physical fatigue and mental fatigue after even light exercise.”
The lab discovered MICU1 and MICU2 only recently. So, patients with this disorder had no explanation for their symptoms, until now. “It’s a pretty rare disorder in general,” Kim notes. “But it helps patients to have a diagnosis, at least, and then we can move toward therapeutics.”
More recently, in collaboration with the Seidman Lab, Kim and her colleagues uncovered a link between a MICU2 loss and diastolic dysfunction in mice. Diastolic dysfunction, the heart’s inability to relax between beats, is both common and dangerous. Although this link requires further research, it implies that MICU1 and MICU2 mutations could contribute to a variety of common diseases. “We think that the uniporter will be linked to many common disorders, but,” Kim cautions, “the mechanism of how mitochondrial calcium uptake contributes to these disorders really needs to be worked out.”
Regulation of the uniporter by calcium___"The molecular era of the mitochondrial calcium uniporter"Nature Reviews Molecular Cell Biology, 2015 |
|
In yet another study, the lab uncovered one more unusual correlation: MICU1 and manganese toxicity. Cells without MICU1 are sensitive to manganese toxicity. Cells without the entire channel, however, are more resistant. “That’s because manganese can get through the channel if cells don’t have MICU1 but, of course, it can’t get through the channel if cells don’t have the channel,” explains Kim. These results may have implications for patients with MICU1 deficiency, and furthermore, may suggest the mitochondria and uniporter have a broader role in Manganism.
During her graduate years in the Mootha Lab, Kim contributed to thirteen papers (four as first author) and another nine as an undergraduate (again, two as first or co-first author). As an undergraduate at the University of Wisconsin-Madison, Kim worked with Dr. Ron Raines in bio-organic chemistry. The experience, she says, “really inspired me to continue doing research.”
Her motivation continued—and continues—to grow. To celebrate her defense, which one committee member called “remarkable,” Kim took a weekend to hike in the White Mountains. But she couldn’t tell you which mountain. And in conversation, at the very least, she prefers the lab to anything else. She’s comfortable there. It’s her world.
After graduation, which she plans to skip, Kim means to continue her exploration of MICU1 and MICU2. Eventually, she hopes to develop new therapeutics for those with MICU-related conditions, perhaps in her own biotech start-up.
“There’s a lot left to be done in this world.” She’s referring to the MICU world, of course. Her world.
Cover illustration artist: Leah Bury