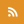More affordable, accurate, and scalable, the test could make it possible to perform population-wide screening
By Caitlin McDermott-Murphy
In the early days of the COVID-19 pandemic, most of Eric Fischer’s researchers were home in self-quarantine. But those permitted to work shared space at the Dana-Farber Cancer Institute with doctors, nurses, and patients, who were potentially COVID-19 positive. So, Fischer thought, why not test the Institute’s employees for antibodies?
But he ran into a problem: Current serological tests that detect COVID-19 antibodies are almost all based on a technology called ELISA, an expensive and time-consuming test with multiple complex steps. “I realized that ELISA is a pretty ridiculously complicated assay format,” Fischer said.
In March, Fischer, an associate professor at the Harvard Medical School, independent investigator and co-director of the Center for Protein Degradation at Dana-Farber Cancer Institute, teamed up with Ralph Mazitschek, an assistant professor at the Harvard Medical School and Massachusetts General Hospital. With the Fischer lab’s expertise in small molecule therapeutics and the Mazitschek group’s knowledge of infectious diseases and testing technology, the team set out to design a simpler, cheaper, and scalable test.
A few months later, they did just that: One of their tests, the duo estimated, would cost just one dollar to perform. And, Mazitschek said, they already have enough to test every single person once in the U.S. The cost and simplicity of their assay could make it possible for labs across the globe, even those with few resources, to perform population-wide screening for COVID-19 antibodies. And, when vaccines start to emerge, they could help monitor their effectiveness.
“This has a real chance of becoming a standard way of testing for COVID antibodies,” said Mazitschek.
The team reported their results in a pre-print this September; the work is now undergoing peer review.

|
LEFT: A light pulse signals to the researchers that a COVID-19 antibody is present RIGHT: The team's "shake-and-bake" method requires far fewer steps (and introduces less room for human error). Photo courtesy of the Mazitschek and Fischer labs |
For their test, the team chose to side-step ELISA technology and improve a different assay called a TR-FRET (Time-Resolved Förster Resonance Energy Transfer). TR-FRET technology is not new. But, until now, the required components were either far too expensive and complicated to make or were not suitable to detect antibodies.
“If you were to buy a milligram of the best performing reagent for these assays, it would cost you around half a million dollars,” said Connor Payne, a Ph.D. candidate in chemistry in the Graduate School of Arts and Sciences and a member of the Mazitschek lab. In the last year, he whittled the 25-step process (which Mazitschek said only the most experienced chemists could perform) to about 11 and developed new, easy-to-access TR-FRET reporter molecules that outperform the commercial versions.
“We pretty much eliminated the cost-prohibitive nature of these reagents while still maintaining their integrity and performance,” said Payne. Now, he can make grams at a time. Sold for market value, those vials are worth a hundred million dollars. Of course, their faster, simpler process will bring that price way, way down.
Two months after Payne perfected the solution, two Fischer lab members—postdoctoral scholar Hong Yue and senior scientist Radoslaw Nowak—worked to incorporate it into a “shake-and-bake” style COVID-19 antibody test.
Instead of the ELISA’s many steps, which slow the process and introduce opportunities for user error, the team’s new test needs just a finger prick worth of diluted patient serum mixed into their solution, incubated for about an hour, and read out in a few seconds. “It really is as simple as that,” Payne said.
Because the ELISA works by increasing the read-out signal, the test is also prone to false positives. Rather than amplifying the signal, the Fischer and Mazitschek team extended the life of their signal by almost a million, eliminating the risk of reading false positives. Without signal amplification, said Fischer, the test could produce false negatives, but they’ve yet to see that happen.
“We want this to be implemented. We understand the potential power behind this technology.” |
- Connor Payne, a Ph.D. candidate in chemistry and member of Mazitschek lab, who is one of the authors on the pre-print |
With the new test, an operator could analyze 400 patient samples in one hour (compared to the ELISA’s 100 samples in one day). To speed the process further, Fischer said, they need a faster way to collect patient samples. “That becomes the bottleneck. It’s no longer the assay.” Next, he hopes to design a method for patients to collect their own samples at home to send in to a lab.
And, as COVID-19 vaccines become available to the general public, the team’s assay could track their effectiveness, measuring post-vaccination antibody levels. Though, Mazitschek said, their current version might need some tweaks to identify different types of antibodies, which, he said, would be as easy as changing the adaptor on a garden hose.
The Mazitschek lab can also easily adapt their technology to search for a vast range of pathogens—like malaria, their original target—beyond the COVID-19 virus. But for now, the pandemic is their top priority.
“We want this to be implemented,” said Payne. “We understand the potential power behind this technology.”