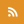Walk outside on a clear summer twilight and you might see hundreds of minute lights spark and glow. Together, fireflies, which produce chemical light through bioluminescence, display a celestial pattern unlike anything on earth. Or, more precisely, unlike anything visible to our unassisted eyes.
In a darkness deeper than those summer twilights, our neurons fire and glow in a similar stellar dance. Now, with Firefly, a new microscope from the Cohen Lab, we can watch neurons pulse, communicate, and shine. The image is at once beautiful and invaluable--it could illuminate how disorders like epilepsy and Alzheimer's disease affect neuron communication and thus enable researchers to discover how to prevent and treat a host of neurological diseases.
The microscope's large field of view and fast imaging capability allows it to image electrical signals quickly traveling from neuron to neuron. Viewing the larger neuronal network is important to understanding how neurological diseases affect neuronal communication. Credit: Daniel Hochbaum, Harvard University
Firefly can resolve 85 neurons in 30 seconds. To accomplish this feat, Professor of Chemistry and Chemical Biology and Physics Adam Cohen and his team exploited a relatively new technique: optogenetics. With optogenetics, a fusion of optics and genetics, researchers modify cells to include genes with light-responsive properties. In this case, Professor Cohen designed neurons to include light-sensitive proteins. Then, the microscope captures sparks of light associated with electrical signals emanating from each neuron, providing precise measurements of their elusive activity.
Although others have produced similar optogenetic tools, Firefly exceeds them in power and range. It can image a 6-millimeter-diameter area, more than one hundred times larger than most analogs. This broad field of vision offers unprecedented views of not just one but hundreds of neurons engaged in communication. Now, researchers can examine when and why communication fails. "This optical system provides a million inputs and a million outputs, allowing us to see everything that's going on in these neural cultures," explained Cohen. "The optical system must be highly efficient to detect good signals within a millisecond. A great deal of engineering went into developing optics that can not only image a large area but do so with very high light collection efficiency."

Cohen's research team tested the microscope on not only neurons but cultured heart cells as well. With the microscope, the researchers successfully mapped their pulsing electrical waves. So, while neuroscientists look for clues to treat epilepsy and Alzheimer's disease, cardiologists can examine the electrical signals of abnormal heart rhythms to explore causes and solutions. And, to ensure easy access for a broad research community, Cohen and colleagues assembled Firefly with economical, commercially-available materials.
"The development of new biological sensors has made measurements that were impossible just a few years ago into something routine," said Daniel Hochbaum, a member of the Firefly team and Harvard's Society of Fellows. "This microscope is a brilliant example of such a tool--it will profoundly increase the possibilities of high-throughput and high-content measurements with existing and future sensors of a diverse array of biological functions."
And, the researchers already have plans to expand their field of vision: "The system we developed is designed for looking at a relatively flat sample such as cultured cells," Cohen said. "We are now developing a system to perform optogenetics approaches in intact tissue, which would allow us to look at how these neurons behave in their native context."
Paper: C. Werley, M.-P. Chien, A. E. Cohen, "An ultrawidefield microscope for high-speed fluorescence imaging and targeted optogenetic stimulation," Biomed. Opt. Express, Volume 8, Issue 12, 5794-5813 (2017).