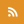How kinks in protein folding could help researchers design new drugs and even fight COVID-19
By Jordan Wilkerson
Right now, your body is making trillions of proteins essential for even basic tasks. You can read this article, for example, thanks to the proteins that make your eye lenses transparent.
Understanding how proteins fold is crucial since the build-up of these misfolded molecules can cause serious health issues in aging human bodies. Misfolded proteins in the eyes, for instance, clump together to form white clouds known as cataracts. In the brain, their accumulation can lead to Parkinson’s and other deadly neurodegenerative diseases. Now, researchers have developed a new technique that reveals for the first time why periodic pausing helps proteins fold properly. This discovery could lead to new drugs preventing disease-causing misfolds or even targeting viruses like SARS-CoV-2 (the novel coronavirus) by weaponizing these errors against them.
All proteins are made up of amino acids that link together like a long chain and fold into a series of helices, pleated sheets, and sharp turns. For a protein to work properly, the way it’s folded is as crucial as the amino acids it’s made of. For larger proteins (most proteins in the human body and most other lifeforms), folding into the right orientation isn’t simple. There are countless ways the molecule can twist and turn.
“There’s only one correct way, though,” said Amir Bitran, a Ph.D. candidate in chemistry in the Graduate School of Arts and Sciences. “A big question is, how do proteins get their structure right?”

|
A misfolded state of the E. coli protein, FabG, simulated by the new algorithm developed by the Shakhnovich lab. Image credit: Amir Bitran |
In a paper published in PNAS, Bitran and other researchers in the Shakhnovich lab report that while ribosomes in cells make certain proteins, they periodically pause to allow the molecule to begin folding into the right structure. Without this clever maneuver, the long strings of amino acids could easily tangle into the wrong orientation and become useless debris in the cell. These pauses are timed to prevent the formation of particularly troublesome misfolded structures — ones that cannot easily refold into the right shape.
Based on a sophisticated new algorithm that Bitran and his colleagues designed, their computer program simulates how all the protein’s atoms — which can reach the thousands — interact with one another to fold the protein while the ribosome pauses. If the ribosome does not take these breaks, the team said, larger proteins can fold into the wrong structures.
To confirm their findings, the authors simulated the stepwise folding of six proteins commonly found in E. coli bacteria. Because its genetics is very well understood, E. coli makes for a great, data-rich model to better understand protein synthesis. But the insights from their algorithm can easily be translated to how proteins are folded in more advanced organisms like humans.
The new technique helped the biochemists “understand what makes proteins misfold and what kind of structures cause long-lived misfolded states,” said Eugene Shakhnovich, a professor of chemistry and chemical biology. Their current technique is built on top of a protein folding algorithm developed by the research group a decade ago. At that time, only small proteins less than 100 amino acids long could be studied. Typically, proteins that small don’t need the ribosome to take breaks while making them. Tiny proteins usually just fold into the right structure without intervention. The latest upgrade “allows us to extend the range of protein lengths and explore the landscape of possible protein structures,” Shakhnovich said. Prior to this work, there was no clear-cut way of determining exactly what a larger protein’s misfolded states might be.
Like all computer simulations, the model is not the same as real life. Though it cannot capture everything, not everything needs to be captured by a computer model: “There’s going to be a ton of things in the cell that affect the details,” said Bitran. “But I think the essence is captured by this simple picture.” To make sure, they have begun studying the processes in real E. coli to test their simulated predictions. The preliminary results look promising so far, suggesting that the model’s simple picture still accurately depicts how proteins get folded — or misfolded.
“That’s exactly what we’re doing for COVID-19 research: developing small molecules that prevent COVID-19 proteins from folding in human cells" |
- Eugene Shakhnovich |
The team’s technique could also be used as a novel tool for designing drugs that make viral proteins fold incorrectly. The scientists have already begun applying their new model to SARS-CoV-2 (the novel coronavirus) with this goal in mind. Their focus is on the spiky proteins that coat the virus surface and allow the pathogen to latch onto human cells. With their new computer model, the team can identify the spiky protein’s potential misfolded states.
“Since we can predict the structure, we can design small molecules to target it,” Shakhnovich said. The small molecule drugs could latch onto misfolded versions and keep them from refolding to the correct shape. “That’s exactly what we’re doing for COVID-19 research: developing small molecules that prevent COVID-19 proteins from folding in human cells.”
Understanding how proteins fold could also help us better understand why the process goes wrong as we age. Just as drugs can be designed to force viral proteins to misfold, the new technique could help ensure our proteins get the right fold.